The “ABCs” of Medical Technology Reimbursement in a Hospital OUTPATIENT Setting
Larry Yost | September 1, 2023 | Blog Post
As I indicated in my previous post, coding is the link between coverage and payment. Coding provides a mechanism for payers and providers to identify diagnoses, medical services, procedures, drugs, devices, laboratory tests, and supplies. Codes are used on claim forms by hospitals, physicians and other healthcare provides to enable claims to be processed and paid more efficiently. The types of codes used vary by provider and by location of service. The following is a brief guide to codes and reimbursement-related classifications used by hospitals for inpatient admissions. While there are some exceptions to what is outlined below, the majority of healthcare services provided by non-governmental hospitals in the U.S. fall under this reimbursement system.
Inpatient care is typically defined as a hospital stay needing 2 or more midnights of medically necessary hospital care. Under this scenario, most hospital are paid based on a DRG or “Diagnostic Related Group” basis. This results in most hospitals receiving a single payment per hospital admission which is designed to cover all services provided (including device and technology costs) during the admission. The DRG the patient falls under is determined by the reason for admission (diagnosis), and in some cases, the procedure performed. When more there is more than one diagnosis or procedure performed, the one that is associated with the highest reimbursement will determine the DRG the patient will be classified under.
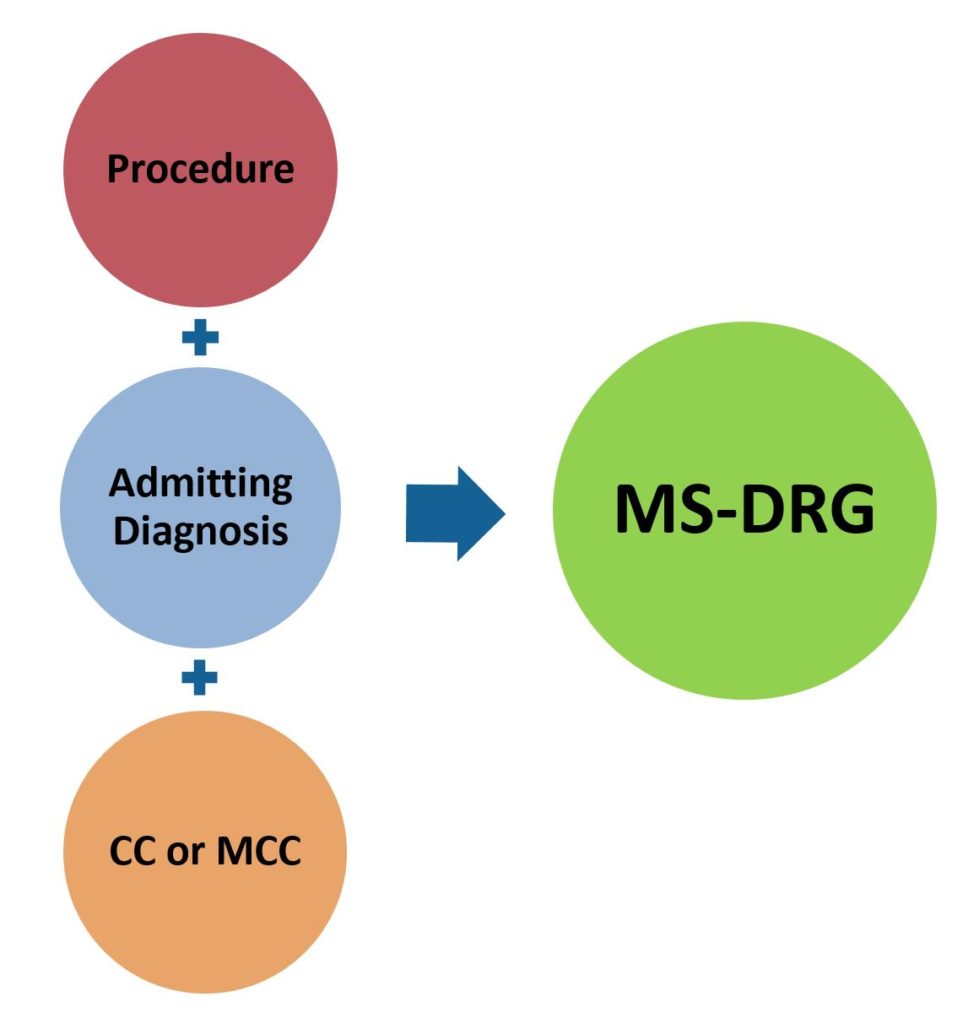 Diagnosis (admission) codes or CD-10-CM Medical Diagnosis Codes are based on the “International Classification of Diseases; 10th Revision” which identify specific codes associated with diseases or conditions which describe the need for medical services. A primary ICD-10 diagnosis code is listed on the claim form along with all secondary ICD-10 diagnosis codes. A listing of current ICD-10 codes, by diseases and conditions, can be found at link. The ICD-10 coding system also has “procedure codes” which are used in conjunction with diagnosis codes to determine DRG classification for inpatient admissions. A listing of ICD-10-PCS 109 procedures codes can be found at link. DRG classification can be based on a primary diagnosis only or on the combination of a diagnosis and an associated procedure. Medicare transitioned from ICD-9 to ICD-10 on October 1, 2015.
Diagnosis (admission) codes or CD-10-CM Medical Diagnosis Codes are based on the “International Classification of Diseases; 10th Revision” which identify specific codes associated with diseases or conditions which describe the need for medical services. A primary ICD-10 diagnosis code is listed on the claim form along with all secondary ICD-10 diagnosis codes. A listing of current ICD-10 codes, by diseases and conditions, can be found at link. The ICD-10 coding system also has “procedure codes” which are used in conjunction with diagnosis codes to determine DRG classification for inpatient admissions. A listing of ICD-10-PCS 109 procedures codes can be found at link. DRG classification can be based on a primary diagnosis only or on the combination of a diagnosis and an associated procedure. Medicare transitioned from ICD-9 to ICD-10 on October 1, 2015.
Medicare transitioned to the MS-DRG or “Medicare Severity Diagnosis Related Groups” in 2008 in which reimbursement levels are adjusted if certain complications/ comorbidities (CCs) or major complications/ comorbidities (MCCs) are present at admission. The MS-DRG system significantly expanded the number of “DRGs” since many previous DRG groups were expanded to include separate DRGs for patients with CCs and/or MCCs. There are now over 740 separate MS-DRGs. There are numerous providers of DRG handbooks which list each MS-DRG and their associated ICD-10 diagnosis/procedure codes. My preference is the DRG Expert by Optum 360 (which I purchase each year). An abbreviated list of Medicare identified CCs and MCCs can be found at link.
I plan to devote a future blog posts to providing insight on CMS’ New Technology Add-On Payment (NTAP) program which provides incremental Medicare payment on top of DRGs for qualifying technologies and a separate post explaining how Medicare determines hospital specific reimbursement rates based on MS-DRG classifications. My next post will focus on codes and reimbursement-related classifications used by hospitals and providers when billing for outpatient admissions or services provided in a ambulatory surgery center.
